Draw The Electron Configuration For A Neutral Atom Of Nickel
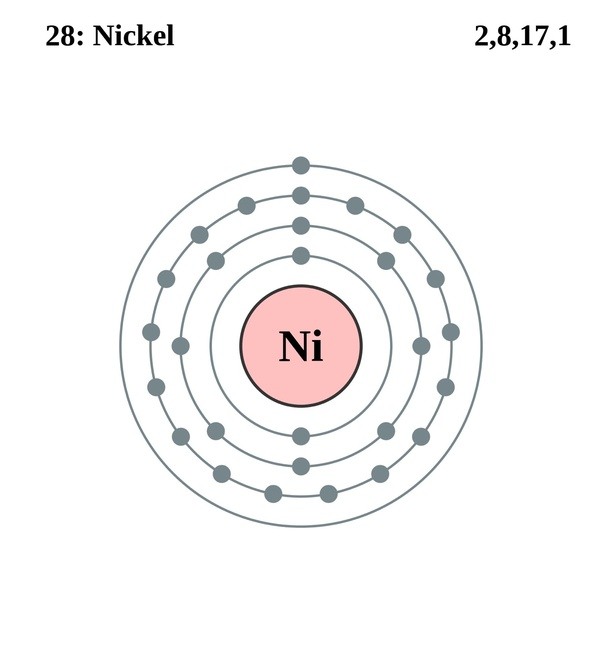
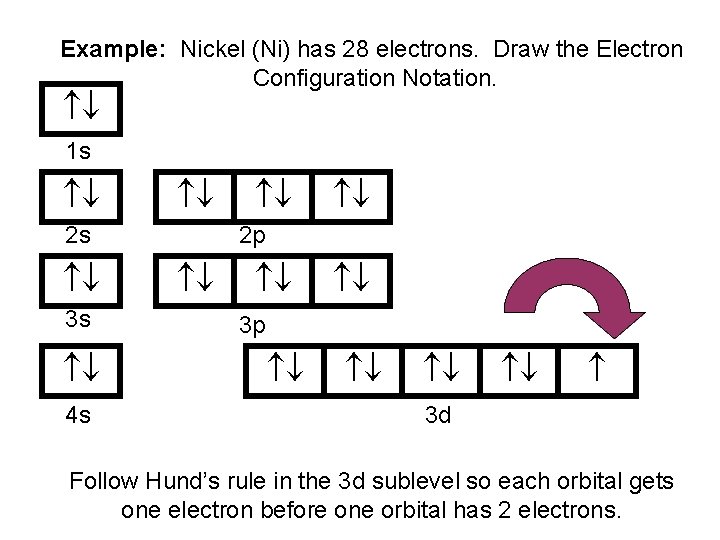
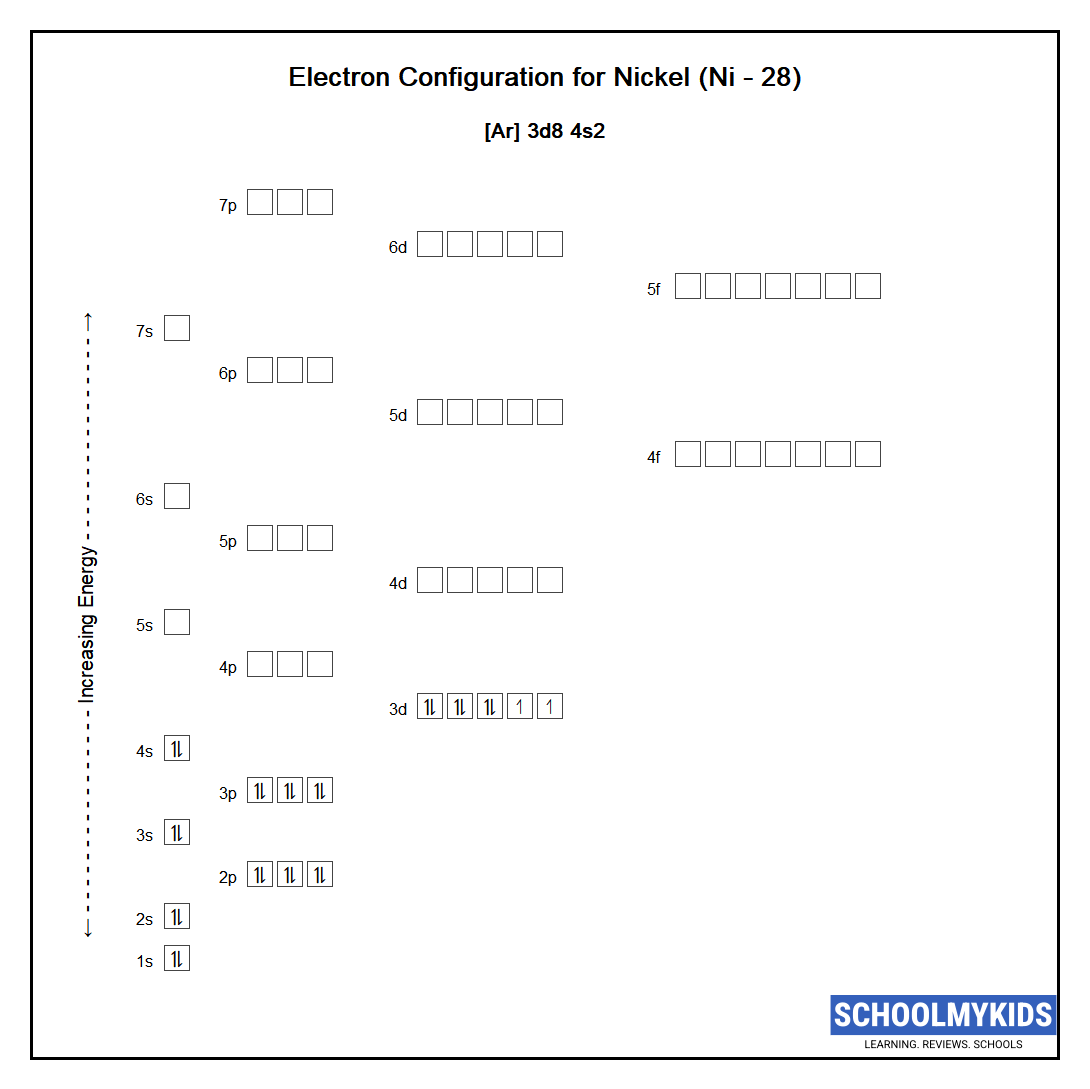
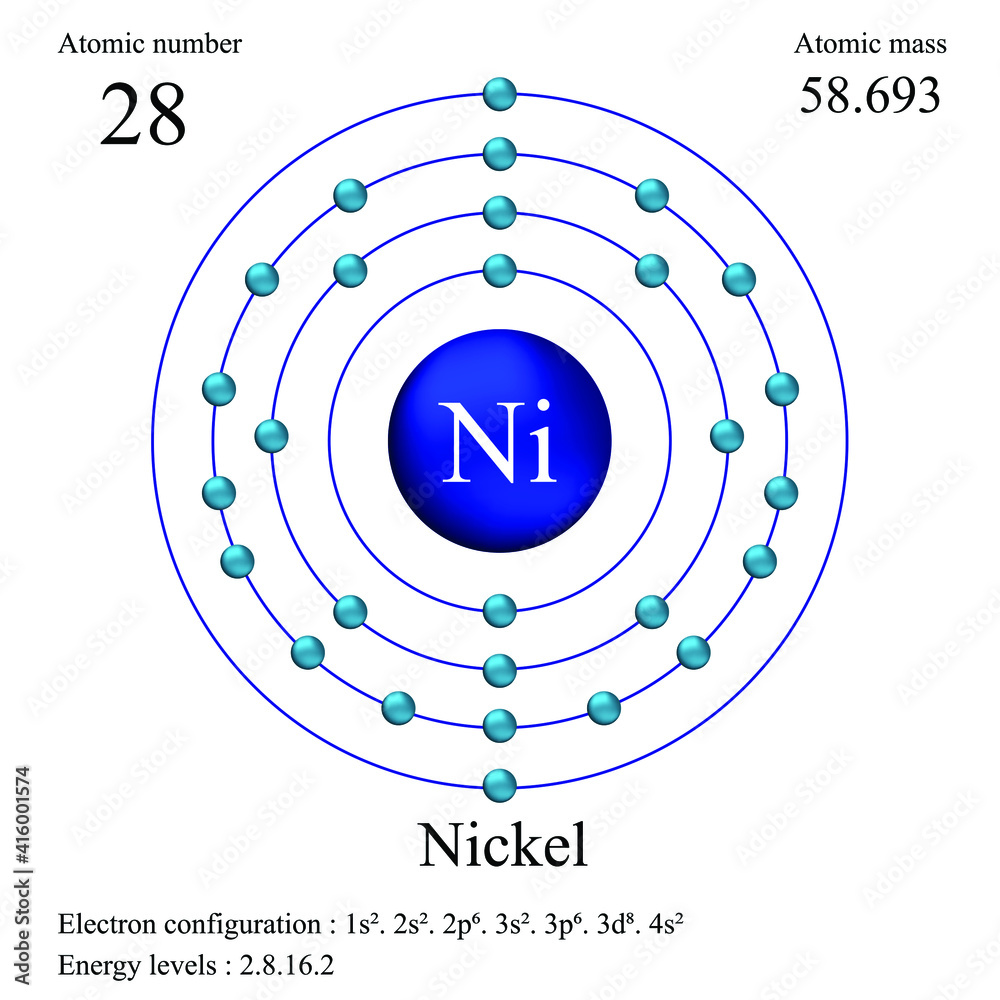
Draw The Electron Configuration For A Neutral Atom Of Nickel - Web click here:point_up_2:to get an answer to your question :writing_hand:how do you write the electron configuration for nickel. The neutral nickel atom therefore must have 2 8 electrons to accommodate according to the usual. Web a nickel nucleus has 28 positively charged nuclear particles, 28 protons. Web ni electronic configuration: Web to write the configuration for the nickel ions, first we need to write the electron configuration for just nickel (ni). The first three (n, l, and m l) may be the same,. Web to write an electron configuration for a cation, start by writing the electron configuration for the neutral atom. Web a neutral helium atom, with an atomic number of 2. The neutral nickel atom therefore must have 28 electrons to accommodate according. Determine how many electrons were lost. We describe an electron configuration with a symbol that. [ar], 3d8, 4s2 atomic number of nickel (ni) = 28 electronic configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d8 or [ar], 3d8, 4s2 where. Web the ground state electron configuration of nickel is [ar].3d8.4s2. Web the same rule will apply to transition metals when forming ions. Following the electron configuration rules, such. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We first need to find the number of. Web ni electronic configuration: To find the electron configuration. Web the ground state electron configuration of a neutral nickel atom is #[ar]3d^84s^2#.this data comes from the nist atomic spectra database. Web an atom's ground state electron configuration describes how the electrons have distributed among the orbital shells and subshells. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁸ so, the electron configuration for a neutral atom of nickel is $\boxed{1s^2 \ 2s^2 \ 2p^6 \. Web a nickel nucleus has 28 positively charged nuclear particles, 28 protons. [ar], 3d8, 4s2 atomic number. We describe an electron configuration with a symbol that. Web write the configuration of the neutral atom. Determine how many electrons were lost. Following the electron configuration rules, such. Web click here:point_up_2:to get an answer to your question :writing_hand:how do you write the electron configuration for nickel. Web to write the configuration for the nickel ions, first we need to write the electron configuration for just nickel (ni). Web assigning electron configuration. Web the electron configuration for nickel is: Write the expanded and shortened. The neutral nickel atom therefore must have 2 8 electrons to accommodate according to the usual. Web the correct electron configuration for a neutral atom of nickel is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁸, which corresponds to option b. We describe an electron configuration with a symbol that. We first need to find the number of. To find the electron configuration. Web an atom's ground state electron configuration describes how the electrons have distributed among. Web an atom's ground state electron configuration describes how the electrons have distributed among the orbital shells and subshells. [ar], 3d8, 4s2 atomic number of nickel (ni) = 28 electronic configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d8 or [ar], 3d8, 4s2 where. Web nickel is in the #4th# energy level, #d# block, #7th# column, this means that the. We describe an electron configuration with a symbol that. For example, the electron configuration of lithium, 1s²2s¹,. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁸ so, the electron configuration for a neutral atom of nickel is $\boxed{1s^2 \ 2s^2 \ 2p^6 \. Web a nickel nucleus has 28 positively charged nuclear particles, 28 protons. Web the ground state electron configuration of. Web electron configurations describe where electrons are located around the nucleus of an atom. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Web a neutral helium atom, with an atomic number of 2. [ar], 3d8, 4s2 atomic number of nickel (ni) = 28 electronic configuration is 1s2, 2s2, 2p6,. The first three (n, l, and m l) may be the same,. Web to write an electron configuration for a cation, start by writing the electron configuration for the neutral atom. Then determine the number of electrons. Web the ground state electron configuration of a neutral nickel atom is #[ar]3d^84s^2#.this data comes from the nist atomic spectra database. 1s² 2s². We describe an electron configuration with a symbol that. [ar], 3d8, 4s2 atomic number of nickel (ni) = 28 electronic configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d8 or [ar], 3d8, 4s2 where. The pauli exclusion principle states that no two electrons can have the same four quantum numbers. For example, the electron configuration of lithium, 1s²2s¹,. Web electron. Web nickel is in the #4th# energy level, #d# block, #7th# column, this means that the electron configuration will end #3d^8# with the #d# orbital being one level lower. Web an atom's ground state electron configuration describes how the electrons have distributed among the orbital shells and subshells. [ar], 3d8, 4s2 atomic number of nickel (ni) = 28 electronic configuration. We write electronic configurations by following the aufbau principle (from german, meaning “building up”). For example, the electron configuration of lithium, 1s²2s¹,. Mastering the electron configuration of nickel ([ar] 3d^8 4s^2) reveals vital insights into its atomic structure. Web the electron configuration for nickel is: The neutral nickel atom therefore must have 2 8 electrons to accommodate according to the usual. Write the expanded and shortened. Web electron configurations describe where electrons are located around the nucleus of an atom. The pauli exclusion principle states that no two electrons can have the same four quantum numbers. Web to write an electron configuration for a cation, start by writing the electron configuration for the neutral atom. Determine how many electrons were lost. We describe an electron configuration with a symbol that. Web nickel is in the #4th# energy level, #d# block, #7th# column, this means that the electron configuration will end #3d^8# with the #d# orbital being one level lower. We first need to find the number of. Web the ground state electron configuration of a neutral nickel atom is #[ar]3d^84s^2#.this data comes from the nist atomic spectra database. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁸ so, the electron configuration for a neutral atom of nickel is $\boxed{1s^2 \ 2s^2 \ 2p^6 \. To find the electron configuration.Electron Configuration For Nickel cloudshareinfo
Nickel(Ni) electron configuration and orbital diagram
Nickel Electron Configuration(Explained for Beginners)
Symbol and electron diagram for nickel Royalty Free Vector
Nickel Electron Configuration (Ni) with Orbital Diagram
Electron Configuration Chapter 5 Electrons have 3 levels
Nickel (Ni) Element Information, Facts, Properties, Uses Periodic
Nickel Atom Science Notes and Projects
Nickel atomic structure has atomic number, atomic mass, electron
Electron Configuration For Nickel cloudshareinfo
Web A Neutral Helium Atom, With An Atomic Number Of 2.
Web Ni Electronic Configuration:
Web The Correct Electron Configuration For A Neutral Atom Of Nickel Is 1S² 2S² 2P⁶ 3S² 3P⁶ 4S² 3D⁸, Which Corresponds To Option B.
1S 2 2S 2 2P 6 3 S 2 3P 6 4S 2 3D 3;
Related Post: